null
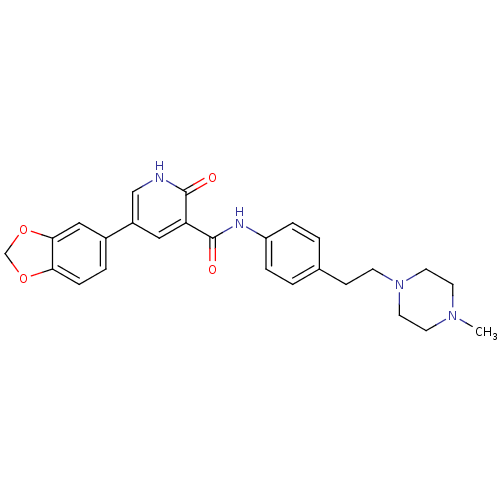
SMILES CN1CCN(CCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1
InChI Key InChIKey=JNAZGEGXKHNDBG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50181672
Found 9 hits for monomerid = 50181672
TargetBreakpoint cluster region protein/Tyrosine-protein kinase ABL1(Homo sapiens (Human))
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Antiproliferative activity against human BCR-ABL positive chronic myeloid leukemia K562 cell lineMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor/Nucleophosmin(Homo sapiens (Human))
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
Affinity DataIC50: 7.50E+3nMAssay Description:Antiproliferative activity against human NPM-ALK positive anaplastic large cell lymphoma karpas299 cell lineMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibitory activity against ALKMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor/Nucleophosmin(Homo sapiens (Human))
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
Affinity DataIC50: 1.74E+4nMAssay Description:Antiproliferative activity against murine BaF3 cell line expressing NPM-ALKMore data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Homo sapiens (Human))
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibitory activity against IGF1RMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibitory activity against Flt3More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity against SrcMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity against AblMore data for this Ligand-Target Pair
TargetInsulin receptor(Homo sapiens (Human))
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibitory activity against IRKMore data for this Ligand-Target Pair