null
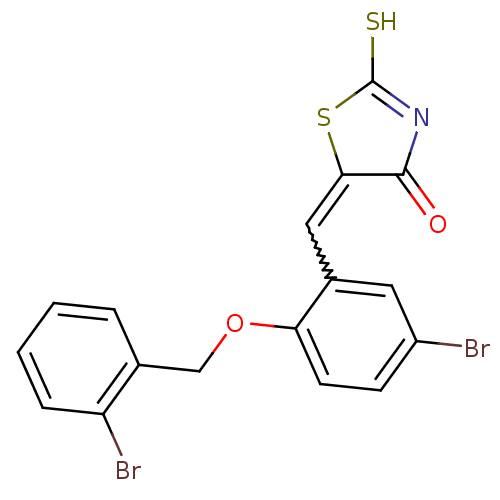
SMILES SC1=NC(=O)C(S1)=Cc1cc(Br)ccc1OCc1ccccc1Br
InChI Key InChIKey=HXNBAOLVPAWYLT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50184288
Found 3 hits for monomerid = 50184288
TargetProtein tyrosine phosphatase type IVA 3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Inhibition of human recombinant PRL-3More data for this Ligand-Target Pair
TargetProtein tyrosine phosphatase type IVA 3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human His6-tagged full length PRL3 assessed as inhibition of DiFMUP dephosphorylationMore data for this Ligand-Target Pair
TargetProtein tyrosine phosphatase type IVA 3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Korea Research Institute of Chemical Technology
Curated by ChEMBL