null
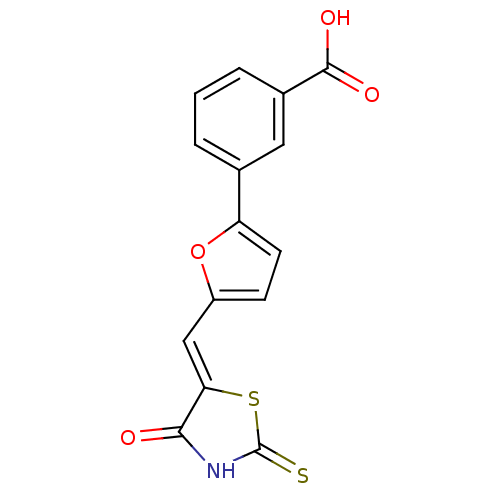
SMILES OC(=O)c1cccc(c1)-c1ccc(\C=C2/SC(=S)NC2=O)o1
InChI Key InChIKey=BHFPIPOWNHCUDR-GHXNOFRVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50189763
Found 3 hits for monomerid = 50189763
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
The University of Jordan
Curated by ChEMBL
The University of Jordan
Curated by ChEMBL
Affinity DataIC50: 920nMAssay Description:Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrateMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
The University of Jordan
Curated by ChEMBL
The University of Jordan
Curated by ChEMBL
Affinity DataIC50: 920nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair