null
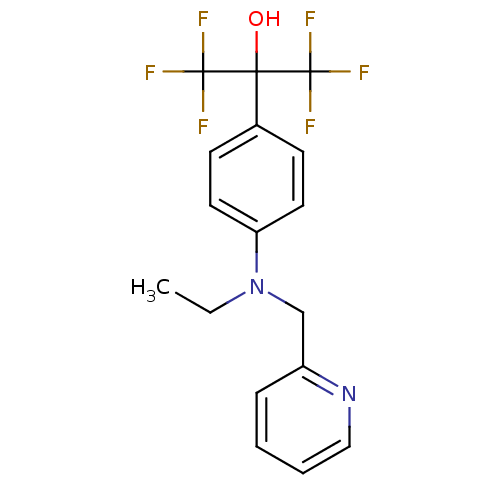
SMILES CCN(Cc1ccccn1)c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F
InChI Key InChIKey=DKNJQXGMJWCWEW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50192155
Found 4 hits for monomerid = 50192155
Affinity DataEC50: 300nMAssay Description:Transactivation of LXRbeta by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Binding affinity to LXRalpha by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataIC50: 460nMAssay Description:Binding affinity to LXRbeta by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataEC50: 700nMAssay Description:Transactivation of LXRalpha by luciferase reporter gene assayMore data for this Ligand-Target Pair