null
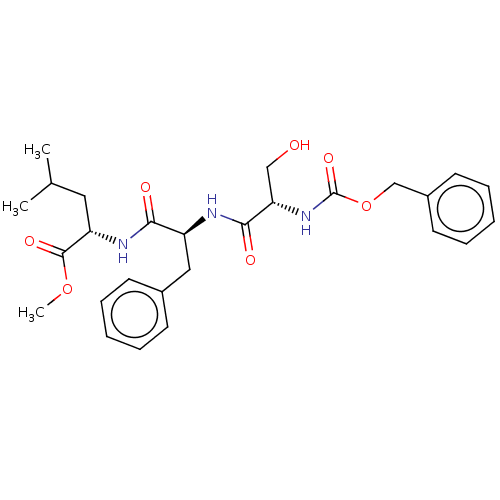
SMILES COC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)OCc1ccccc1
InChI Key InChIKey=HZASFOJVNSQXLC-VABKMULXSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50195933
Found 2 hits for monomerid = 50195933
TargetProteasome subunit beta type-5(Homo sapiens (Human))
University of Naples Federico II
Curated by ChEMBL
University of Naples Federico II
Curated by ChEMBL
Affinity DataKi: 1.03E+4nMAssay Description:Competitive inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-Leu-Leu-Val-Tyr-AMC as substrate measured for 10 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+4nMAssay Description:Inhibition of recombinant HIV1 protease using FRET substrate peptide by FRET assayMore data for this Ligand-Target Pair