null
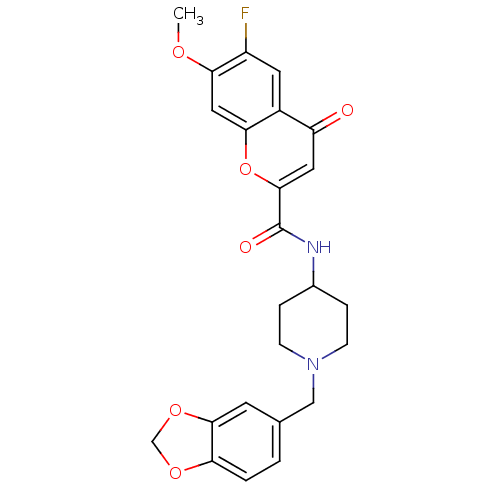
SMILES COc1cc2oc(cc(=O)c2cc1F)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1
InChI Key InChIKey=KESABOVUGAHUQM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50197135
Found 3 hits for monomerid = 50197135
TargetMelanin-concentrating hormone receptor 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [125I]MCH from MCHr1 expressed in IMR32 cellsMore data for this Ligand-Target Pair
TargetMelanin-concentrating hormone receptor 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Antagonist activity at MCHr1 assessed as inhibition of MCH-mediated calcium ion release in intact IMR32 cells by FLIPR assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 15.9nMAssay Description:Displacement of [3H]dofetilide from hERGMore data for this Ligand-Target Pair