null
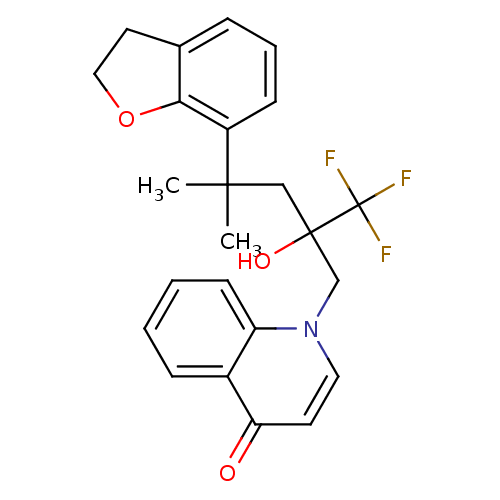
SMILES CC(C)(CC(O)(Cn1ccc(=O)c2ccccc12)C(F)(F)F)c1cccc2CCOc12
InChI Key InChIKey=DKFBPHVUBSXDJB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50201081
Found 3 hits for monomerid = 50201081
TargetMineralocorticoid receptor(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 98nMAssay Description:Inhibition of tetramethylrhodamine labeled dexamethosone binding to MR by FP assayMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assayMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Inhibition of tetramethylrhodamine labeled RU486 binding to PR by FP assayMore data for this Ligand-Target Pair