null
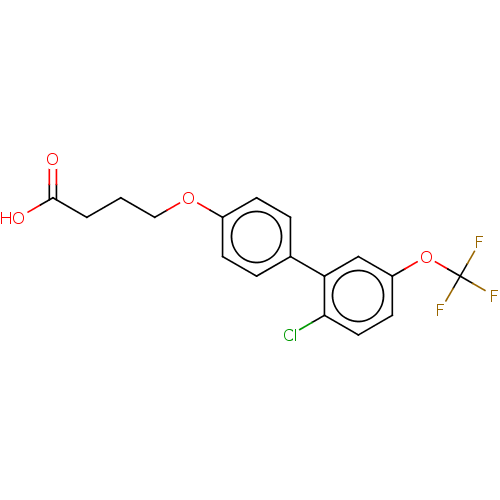
SMILES OC(=O)CCCOc1ccc(cc1)-c1cc(OC(F)(F)F)ccc1Cl
InChI Key InChIKey=FGXVJHYCASUBMZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50203654
Found 9 hits for monomerid = 50203654
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck& Co.
Curated by ChEMBL
Merck& Co.
Curated by ChEMBL
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataEC50: 57nMAssay Description:Agonist activity at human GPR120 expressed in CHOK1 cells measured after 60 mins by IP1-HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 9nMAssay Description:Agonist activity at mouse GPR120 expressed in CHOK1 cells measured after 60 mins by IP1-HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.74E+4nMAssay Description:Inhibition of Nav1.5 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 1.04E+3nMAssay Description:Agonist activity at human GPR40 measured after 60 mins by IP1-HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C(Homo sapiens (Human))
Merck& Co.
Curated by ChEMBL
Merck& Co.
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Cav1.2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair