null
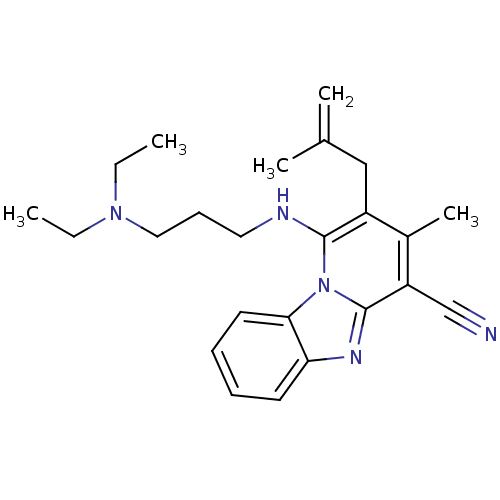
SMILES CCN(CC)CCCNc1c(CC(C)=C)c(C)c(C#N)c2nc3ccccc3n12
InChI Key InChIKey=MTDJLYRNZKODDY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50205732
Found 2 hits for monomerid = 50205732
Affinity DataIC50: 2.24E+4nMAssay Description:Displacement of [125I]MIP1beta from CCR5 expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.90E+3nMAssay Description:Agonist activity at CCR5 expressed in CHO-K1 cells in aqeuorin based assayMore data for this Ligand-Target Pair