null
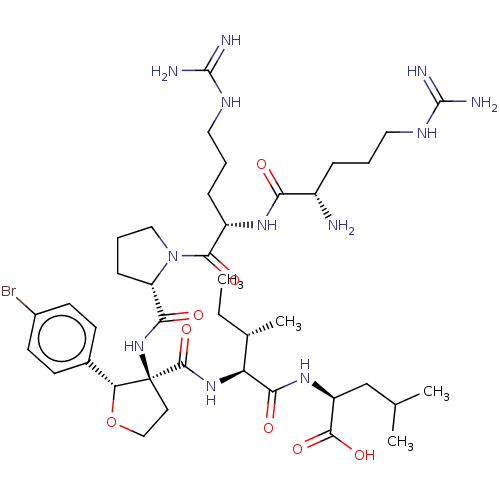
SMILES CC[C@H](C)[C@H](NC(=O)[C@@]1(CCO[C@@H]1c1ccc(Br)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O
InChI Key InChIKey=SNDVFAKLFRYOKC-XMJWERELSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50206708
Found 2 hits for monomerid = 50206708
Affinity DataKi: 615nMAssay Description:Displacement of [leucine-3H]NT (8 to 13 residues) from human NTS2 receptor expressed in HEK293 cell membranes after 60 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Displacement of [3H]neurotensin from human NTS1 receptor expressed in CHOK1 cell membranes after 60 mins by scintillation countingMore data for this Ligand-Target Pair