null
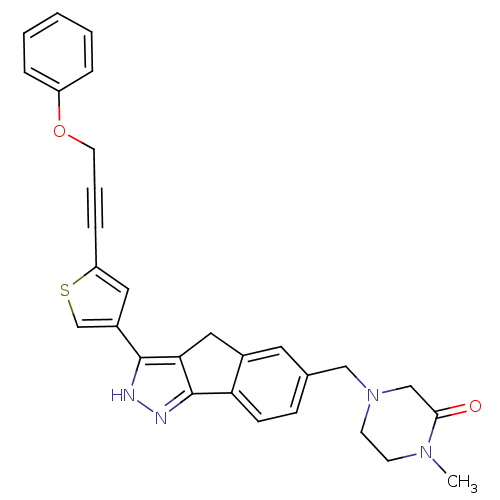
SMILES CN1CCN(Cc2ccc-3c(Cc4c-3n[nH]c4-c3csc(c3)C#CCOc3ccccc3)c2)CC1=O
InChI Key InChIKey=SRMUFZVEAUTYTO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50210285
Found 3 hits for monomerid = 50210285
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of human KDR kinase by HTRF assayMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of VEGF-induced phosphorylation of human KDR expressed in mouse NIH3T3 cell line by Western blotMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of hERG expressed in HEK293 cells assessed as effect on ionic current by patch clamp assayMore data for this Ligand-Target Pair