null
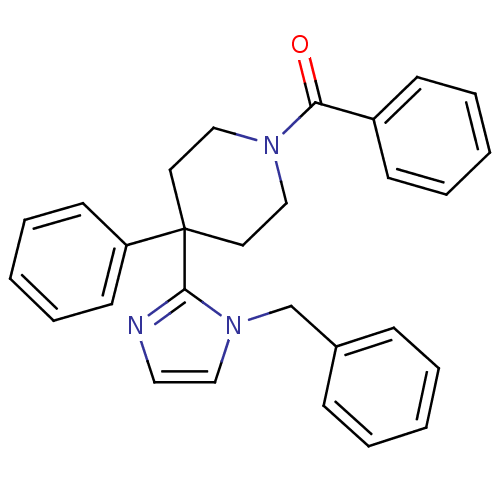
SMILES O=C(N1CCC(CC1)(c1nccn1Cc1ccccc1)c1ccccc1)c1ccccc1
InChI Key InChIKey=XHGVOFUYLQFSIW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50213404
Found 3 hits for monomerid = 50213404
TargetDelta-type opioid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Displacement of [3H]DPDPE from cloned human delta opioid receptorMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 124nMAssay Description:Displacement of [3H]U-69593 from cloned human kappa opioid receptorMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >4.65E+3nMAssay Description:Displacement of [3H]DAMGO from cloned human mu opioid receptorMore data for this Ligand-Target Pair