null
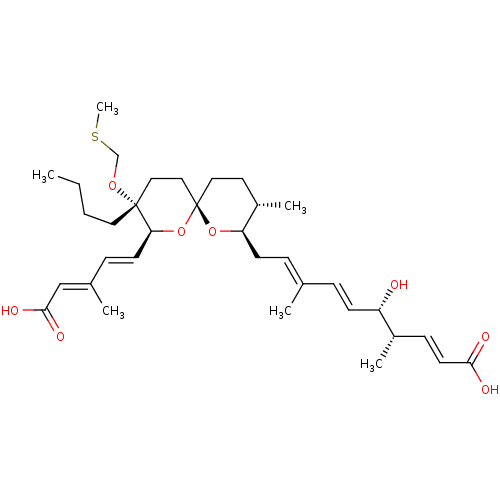
SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OCSC)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O
InChI Key InChIKey=IMGPRJUHDGLGKL-GUAVJJNGSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50217839
Found 2 hits for monomerid = 50217839
TargetIsoleucine--tRNA ligase, cytoplasmic(Homo sapiens (Human))
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
TargetIsoleucyl-tRNA synthetase(Rattus norvegicus)
RIKEN (The Institute of Physical and Chemical Research)
Curated by ChEMBL
RIKEN (The Institute of Physical and Chemical Research)
Curated by ChEMBL
Affinity DataIC50: 353nMAssay Description:Inhibitory concentration against Isoleucyl-tRNA synthetase (IleRS) activityMore data for this Ligand-Target Pair