null
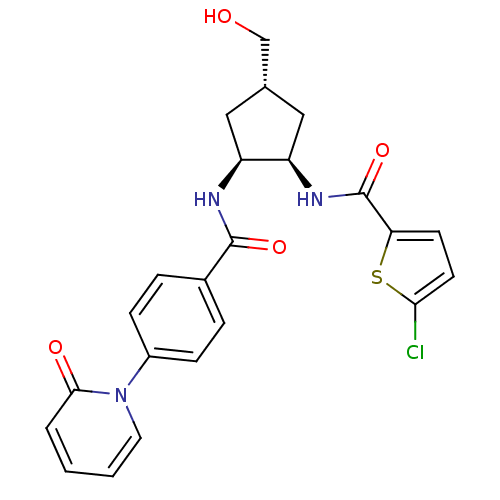
SMILES OC[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O
InChI Key InChIKey=LWARIAPUYJUPKK-JCGIZDLHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50218290
Found 7 hits for monomerid = 50218290
Affinity DataKi: 0.560nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: >1.08E+4nMAssay Description:Inhibition of human plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: >1.10E+4nMAssay Description:Inhibition of human factor 7aMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Bristol-Myers Squibb Company
Curated by ChEMBL
Bristol-Myers Squibb Company
Curated by ChEMBL
Affinity DataKi: >1.40E+4nMAssay Description:Inhibition of human urokinaseMore data for this Ligand-Target Pair
TargetVitamin K-dependent protein C(Homo sapiens (Human))
Bristol-Myers Squibb Company
Curated by ChEMBL
Bristol-Myers Squibb Company
Curated by ChEMBL
Affinity DataKi: >2.10E+4nMAssay Description:Inhibition of human activated protein CMore data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bristol-Myers Squibb Company
Curated by ChEMBL
Bristol-Myers Squibb Company
Curated by ChEMBL
Affinity DataKi: >2.10E+4nMAssay Description:Inhibition of human tissue plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: >2.20E+4nMAssay Description:Inhibition of human PlasminMore data for this Ligand-Target Pair