null
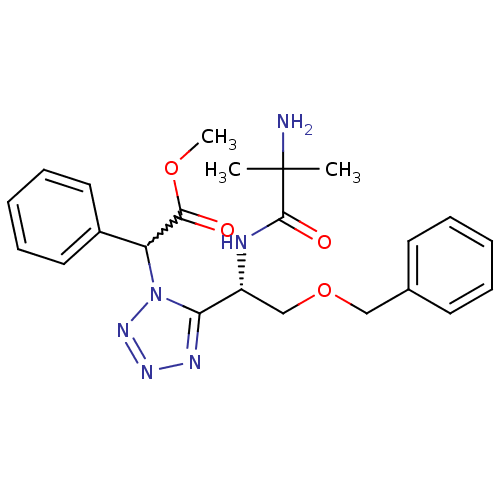
SMILES COC(=O)C(c1ccccc1)n1nnnc1[C@@H](COCc1ccccc1)NC(=O)C(C)(C)N
InChI Key InChIKey=LPWJMAWMFHNOQH-MRTLOADZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50222136
Found 2 hits for monomerid = 50222136
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 470nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cells assessed as intracellular calcium concentration by FLIPR assayMore data for this Ligand-Target Pair