null
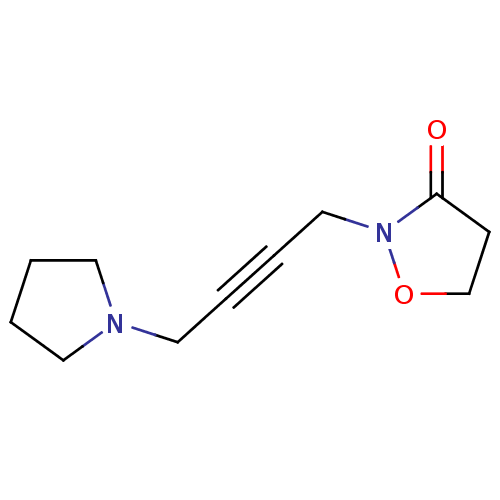
SMILES O=C1CCON1CC#CCN1CCCC1
InChI Key InChIKey=VMJSEWCRPGCNOF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50223182
Found 9 hits for monomerid = 50223182
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Universit£ degli Studi di Milano
Curated by ChEMBL
Universit£ degli Studi di Milano
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:Displacement of [3H]QNB from human muscarinic M2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M4(Homo sapiens (Human))
Universit£ degli Studi di Milano
Curated by ChEMBL
Universit£ degli Studi di Milano
Curated by ChEMBL
Affinity DataKi: 222nMAssay Description:Displacement of [3H]QNB from human muscarinic M4 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Universit£ degli Studi di Milano
Curated by ChEMBL
Universit£ degli Studi di Milano
Curated by ChEMBL
Affinity DataKi: 392nMAssay Description:Displacement of [3H]QNB from human muscarinic M3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
Universit£ degli Studi di Milano
Curated by ChEMBL
Universit£ degli Studi di Milano
Curated by ChEMBL
Affinity DataKi: 510nMAssay Description:Displacement of [3H]QNB from human muscarinic M5 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Universit£ degli Studi di Milano
Curated by ChEMBL
Universit£ degli Studi di Milano
Curated by ChEMBL
Affinity DataKi: 905nMAssay Description:Displacement of [3H]QNB from human muscarinic M1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:Binding affinity against Muscarinic receptor M1 in rat brain using [3H]-PZ (pirenzepine) radioligand at a concentration of 1 nMMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Binding affinity against Muscarinic receptor M2 in rat brain using [3H]-QNB (quinuclidinyl benzylate) radioligand at a concentration of 0.12 nMMore data for this Ligand-Target Pair
Affinity DataIC50: 215nMAssay Description:Muscarinic receptor M2 in rat heart using [3H]-QNB (quinuclidinyl benzylate) radioligand as a M2 non-selective muscarinic receptor antagonist at a co...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Binding affinity against Muscarinic receptor M2 in rat brain using [3H]-QNB (quinuclidinyl benzylate) radioligand at a concentration of 0.12 nMMore data for this Ligand-Target Pair