null
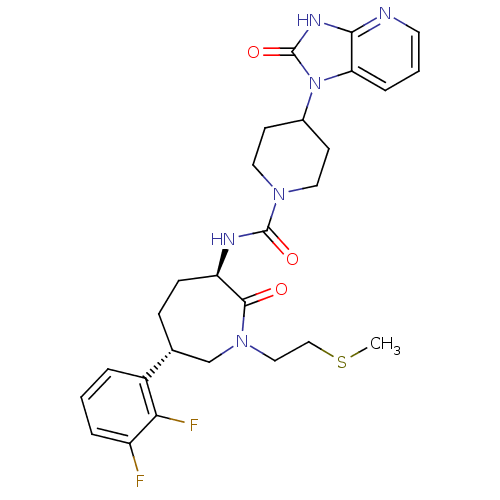
SMILES CSCCN1C[C@@H](CC[C@@H](NC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)C1=O)c1cccc(F)c1F
InChI Key InChIKey=FXGAKFCCNIIEPA-DYESRHJHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50224422
Found 3 hits for monomerid = 50224422
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Displacement of [125I]CGPR from human CL receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Antagonist activity at human CL receptor expressed in E10 cells assessed as CGRP-stimulated cAMP productionMore data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Antagonist activity at human CL receptor expressed in E10 cells assessed as CGRP-stimulated cAMP production in presence of 50% human serumMore data for this Ligand-Target Pair