null
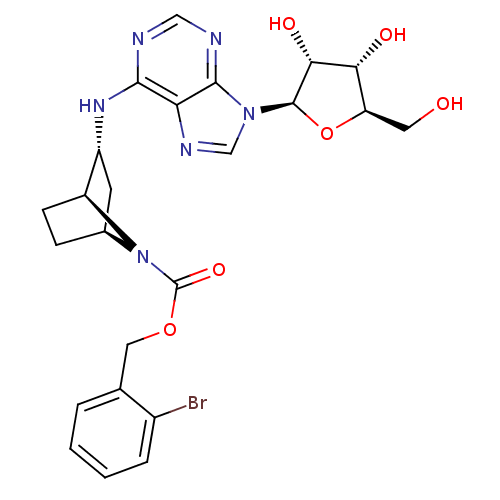
SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3C[C@@H]4CC[C@@H]3N4C(=O)OCc3ccccc3Br)ncnc12
InChI Key InChIKey=IZZJDWPOHUZREF-YXHKVSNWSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50225881
Found 3 hits for monomerid = 50225881
TargetAdenosine receptor A1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 21nMAssay Description:Displacement of [3H]CPX from adenosine A1 receptor expressed in DDT1 MF2 cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]ZM-241385 from adenosine A2A receptor expressed in PC12 cell membraneMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Agonist activity at adenosine A1 receptor expressed in DDT1 MF2 cells assessed as inhibition of (-)-isoproterenol-stimulated cAMP accumulationMore data for this Ligand-Target Pair