null
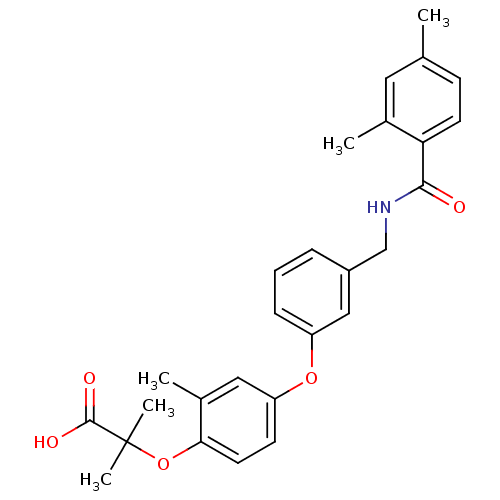
SMILES Cc1ccc(C(=O)NCc2cccc(Oc3ccc(OC(C)(C)C(O)=O)c(C)c3)c2)c(C)c1
InChI Key InChIKey=FGPOTUPYQZSXAV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50227766
Found 12 hits for monomerid = 50227766
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataEC50: 1.04E+3nMAssay Description:Agonist activity at human PPARalpha expressed in CV1 cells by receptor transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataEC50: 25nMAssay Description:Agonist activity at human PPARgamma expressed in CV1 cells by receptor transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Displacement of [3H]-2-methyl-2-(4-{3-{propyl-(5-pyridin-2-yl-thiophene-2-sulfonyl)-amino}-propyl}-phenoxy)-propionic acid from human PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataEC50: 286nMAssay Description:Agonist activity at human PPARdelta expressed in CV1 cells by receptor transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Agonist activity at PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 1.75E+3nMAssay Description:Agonist activity at PPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Agonist activity at PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Agonist activity at PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Agonist activity at PPARgammaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 1.75E+3nMAssay Description:Agonist activity at PPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 1.75E+3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARalphaMore data for this Ligand-Target Pair