null
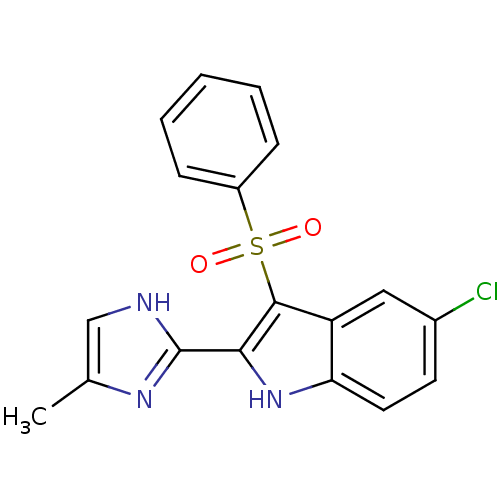
SMILES Cc1c[nH]c(n1)-c1[nH]c2ccc(Cl)cc2c1S(=O)(=O)c1ccccc1
InChI Key InChIKey=YZEAJCOIBSMIOB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50230553
Found 4 hits for monomerid = 50230553
Affinity DataIC50: 9nMAssay Description:Inhibitory activity against K103N mutant of HIV-1 reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of HIV NL43 recombinant reverse transcriptase K103N mutant by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibitory activity against K103N mutant of HIV-1 reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibitory activity against HIV-1 RT with poly.rC-oligo.dG template primerMore data for this Ligand-Target Pair