null
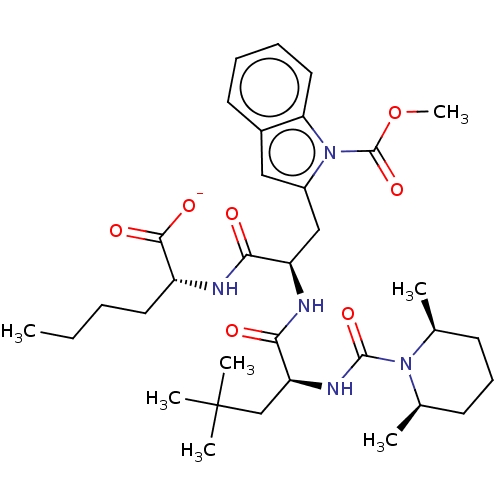
SMILES [Na+].CCCC[C@@H](NC(=O)[C@@H](Cc1cc2ccccc2n1C(=O)OC)NC(=O)[C@H](CC(C)(C)C)NC(=O)N1[C@@H](C)CCC[C@H]1C)C([O-])=O
InChI Key InChIKey=VZGFFFZTTFIUMI-FUKQNADPSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50249565
Found 1 hit for monomerid = 50249565
Affinity DataIC50: 1.20nMAssay Description:Antagonist activity at ETB receptor (unknown origin) expressed in HEK293T cells measured after 30 mins by CCF4-AM dye based GeneBlazer FRET assayMore data for this Ligand-Target Pair