null
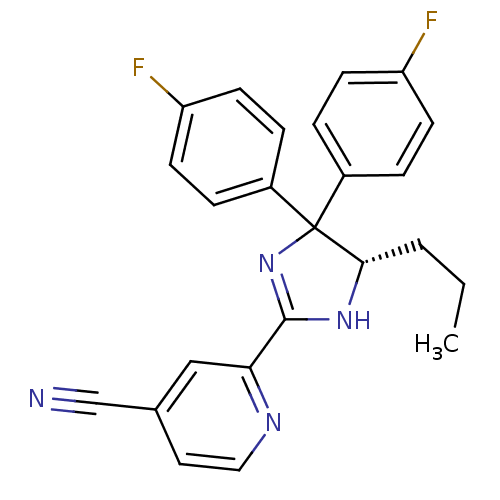
SMILES CCC[C@@H]1NC(=NC1(c1ccc(F)cc1)c1ccc(F)cc1)c1cc(ccn1)C#N
InChI Key InChIKey=ZYAQGVAAYQRPEP-QFIPXVFZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50249755
Found 2 hits for monomerid = 50249755
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataKi: 8.60nMAssay Description:Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 490nMAssay Description:Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]6-yl]methanesu...More data for this Ligand-Target Pair