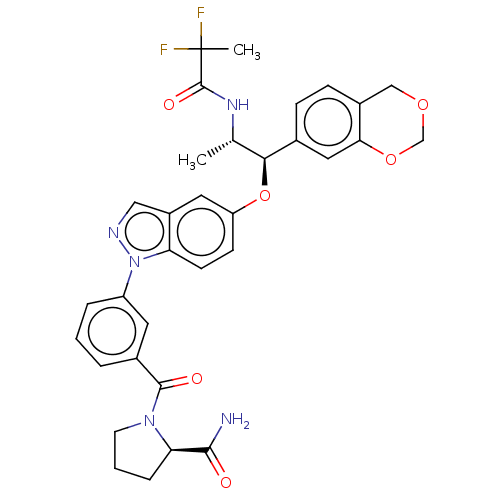
null
SMILES C[C@H](NC(=O)C(C)(F)F)[C@H](Oc1ccc2n(ncc2c1)-c1cccc(c1)C(=O)N1CCC[C@@H]1C(N)=O)c1ccc2COCOc2c1
InChI Key InChIKey=JTXOLPRFYFONSZ-OFIANZKKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50250119
Found 2 hits for monomerid = 50250119
Affinity DataIC50: 0.610nMAssay Description:Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by...More data for this Ligand-Target Pair