null
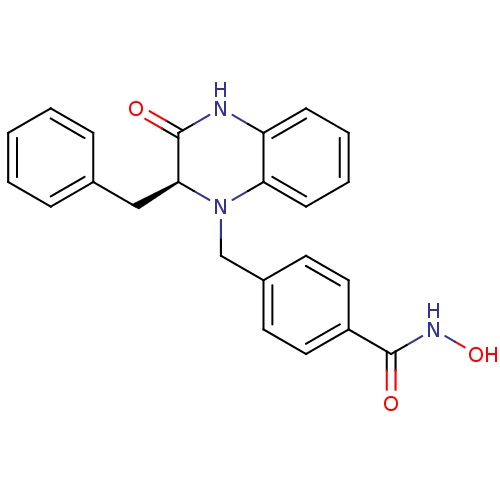
SMILES ONC(=O)c1ccc(CN2[C@@H](Cc3ccccc3)C(=O)Nc3ccccc23)cc1
InChI Key InChIKey=CKLZDMBNAUWUEQ-NRFANRHFSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50255095
Found 3 hits for monomerid = 50255095
Affinity DataIC50: 460nMAssay Description:Inhibition of human recombinant histone deacetylase 2More data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibition of human recombinant histone deacetylase 8More data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibition of human recombinant histone deacetylase 6More data for this Ligand-Target Pair