null
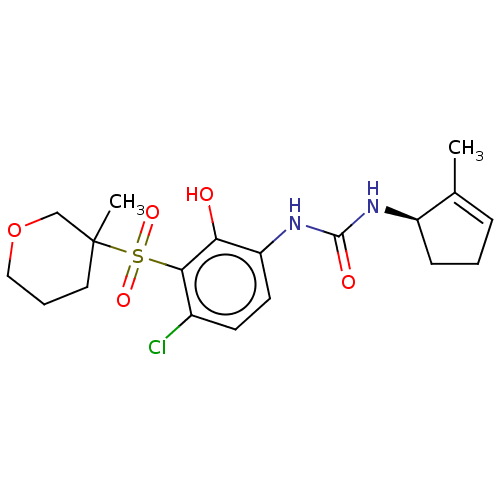
SMILES CC1=CCC[C@H]1NC(=O)Nc1ccc(Cl)c(c1O)S(=O)(=O)C1(C)CCCOC1
InChI Key InChIKey=LUAZWHMHXSLENT-MJTSIZKDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50255563
Found 2 hits for monomerid = 50255563
TargetC-X-C chemokine receptor type 2(Homo sapiens (Human))
Neurosciences Therapeutic Area Unit , GSK Pharmaceuticals R&D , 898 Halei Road, Zhangjiang Hi-Tech Park , Pudong , Shanghai 201203 , P. R. China.
Curated by ChEMBL
Neurosciences Therapeutic Area Unit , GSK Pharmaceuticals R&D , 898 Halei Road, Zhangjiang Hi-Tech Park , Pudong , Shanghai 201203 , P. R. China.
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Antagonist activity at TEV protease cleavage site linked GAL4-VP16-fused recombinant human CXCR2 expressed in cells assessed as inhibition of TEV pro...More data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 2(Homo sapiens (Human))
Neurosciences Therapeutic Area Unit , GSK Pharmaceuticals R&D , 898 Halei Road, Zhangjiang Hi-Tech Park , Pudong , Shanghai 201203 , P. R. China.
Curated by ChEMBL
Neurosciences Therapeutic Area Unit , GSK Pharmaceuticals R&D , 898 Halei Road, Zhangjiang Hi-Tech Park , Pudong , Shanghai 201203 , P. R. China.
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Antagonist activity at CXCR2 in human whole blood assessed as inhibition of GROalpha-stimulated CD11b upregulation preincubated for 15 mins followed ...More data for this Ligand-Target Pair