null
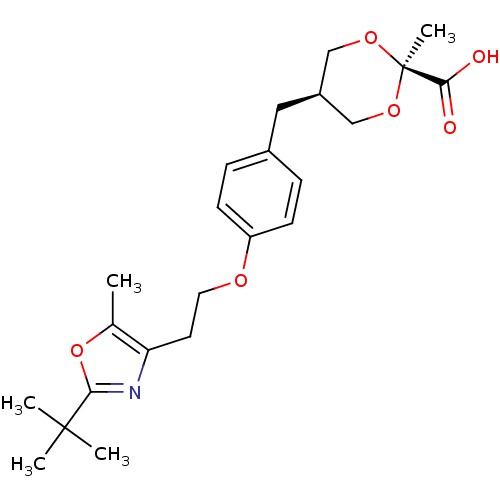
SMILES Cc1oc(nc1CCOc1ccc(C[C@H]2CO[C@](C)(OC2)C(O)=O)cc1)C(C)(C)C
InChI Key InChIKey=KLUBODSSPYNARE-LBEPJEHYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50261880
Found 2 hits for monomerid = 50261880
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Zydus Research Centre
Curated by ChEMBL
Zydus Research Centre
Curated by ChEMBL
Affinity DataEC50: 5.01E+3nMAssay Description:Agonist activity at PPARgamma (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Zydus Research Centre
Curated by ChEMBL
Zydus Research Centre
Curated by ChEMBL
Affinity DataEC50: 4.20nMAssay Description:Agonist activity at PPARalpha (unknown origin) expressed in human HepG2 cells assessed as induction of receptor transactivation by reporter gene assa...More data for this Ligand-Target Pair