null
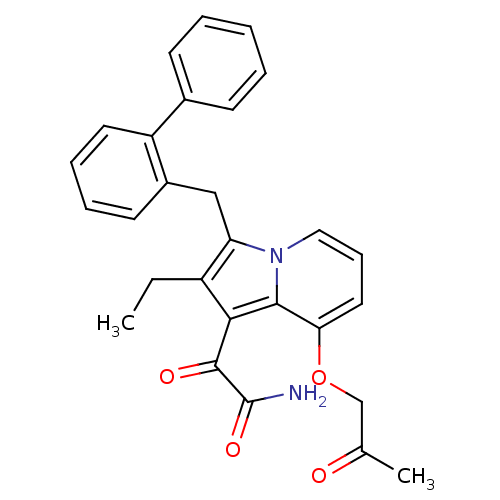
SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(C)=O)c2c1C(=O)C(N)=O
InChI Key InChIKey=SDDRDZUPYOUOOW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50263052
Found 14 hits for monomerid = 50263052
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human group1B phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of mouse group1B phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
TargetPhospholipase A2, membrane associated(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of human group2A phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of mouse group2A phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
TargetGroup IID secretory phospholipase A2(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human group2D phospholipase A2 by [3H]oleic acid-labeled Escherichia coli membrane assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of mouse group2D phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of mouse group2X phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:Inhibition of mouse group2E phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
TargetGroup IIF secretory phospholipase A2(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human group2F phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of mouse group2F phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human group2V phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of mouse group2V phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
TargetGroup 10 secretory phospholipase A2(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human group2X phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair
TargetGroup IIE secretory phospholipase A2(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of human group2E phospholipase A2 fluorimetric assayMore data for this Ligand-Target Pair