null
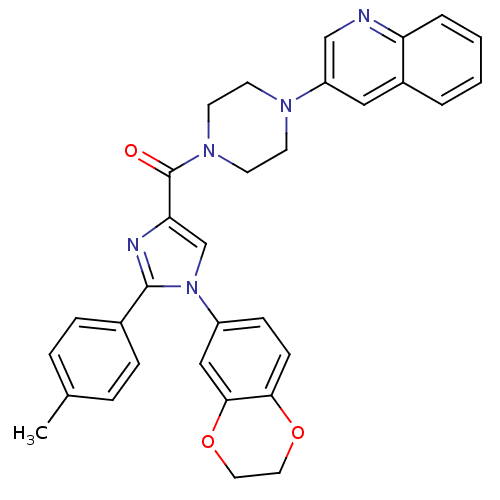
SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1
InChI Key InChIKey=QBDYSDATVFHDRE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50263186
Found 3 hits for monomerid = 50263186
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: 0.120nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human CCK2 receptorMore data for this Ligand-Target Pair