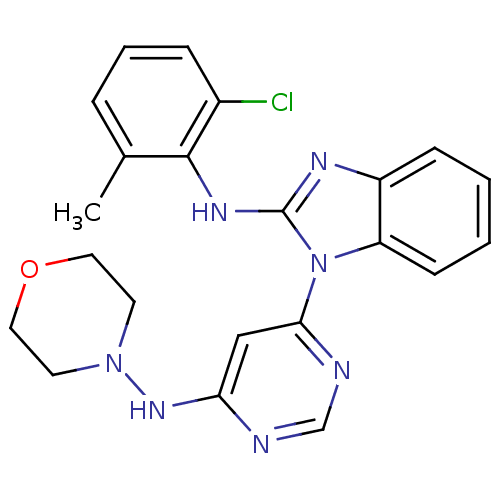
null
SMILES Cc1cccc(Cl)c1Nc1nc2ccccc2n1-c1cc(NN2CCOCC2)ncn1
InChI Key InChIKey=XJMUUEMSIJHHBF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50263475
Found 2 hits for monomerid = 50263475
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of TEL fused Lck (unknown origin)-mediated proliferation of TEL-Lck transformed mouse BA/F3 cells after 48 hrs by bright-glo luciferase as...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of TEL fused Lck (unknown origin)-mediated proliferation of TEL-Lck transformed mouse BA/F3 cells after 48 hrs by bright-glo luciferase as...More data for this Ligand-Target Pair