null
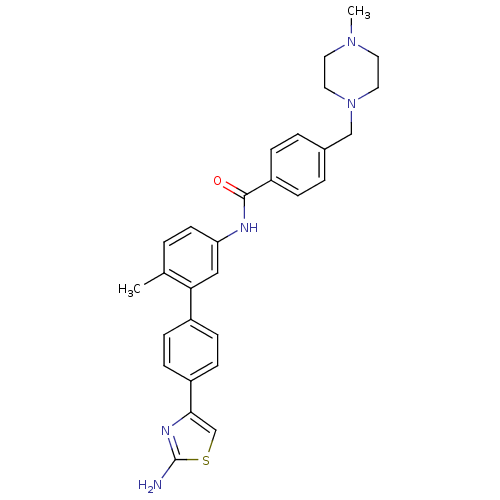
SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(c2)-c2ccc(cc2)-c2csc(N)n2)CC1
InChI Key InChIKey=BGOWMSRXCRUANU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50264990
Found 10 hits for monomerid = 50264990
Affinity DataKi: >850nMAssay Description:Displacement of fluorescent ATP competitive ligand from p38alphaMore data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
GlaxoSmithKline R&D
Curated by ChEMBL
GlaxoSmithKline R&D
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
GlaxoSmithKline R&D
Curated by ChEMBL
GlaxoSmithKline R&D
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of VEGFR2More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of JNK3More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of LCKMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
GlaxoSmithKline R&D
Curated by ChEMBL
GlaxoSmithKline R&D
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of PLK1More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline R&D
Curated by ChEMBL
GlaxoSmithKline R&D
Curated by ChEMBL
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of SGK1More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CDK2More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-4(Homo sapiens (Human))
GlaxoSmithKline R&D
Curated by ChEMBL
GlaxoSmithKline R&D
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of ErbB4More data for this Ligand-Target Pair