null
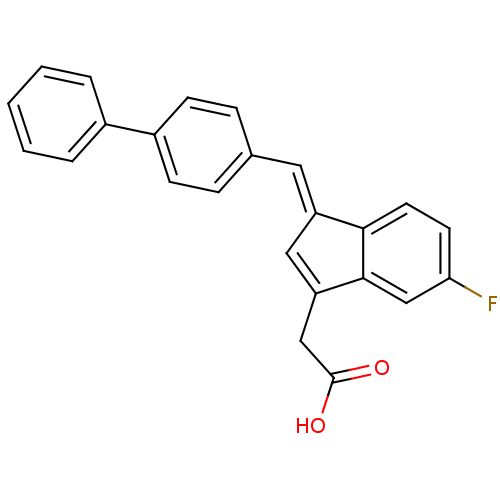
SMILES OC(=O)CC1=C\C(=C/c2ccc(cc2)-c2ccccc2)c2ccc(F)cc12
InChI Key InChIKey=VJDZAFANKNWYHT-XDHOZWIPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50273065
Found 4 hits for monomerid = 50273065
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Vanderbilt University Center for Structural Biology
Curated by ChEMBL
Vanderbilt University Center for Structural Biology
Curated by ChEMBL
Affinity DataEC50: 100nMAssay Description:Agonist activity at PPARgamma expressed in human HCA7 cells assessed as induction of peroxisome proliferator response element after 12 hrs by lucifer...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Mus musculus (Mouse))
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of mouse COX-2 using [14C] arachidonic acid as substrate preincubated for 17 mins before substrate addition measured after 3 mins by thin-...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
Affinity DataIC50: 570nMAssay Description:Inhibition of ovine COX-1 using [14C] arachidonic acid as substrate preincubated for 17 mins before substrate addition measured after 3 mins by thin-...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of COX-1 in human OVCAR3 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured after...More data for this Ligand-Target Pair