null
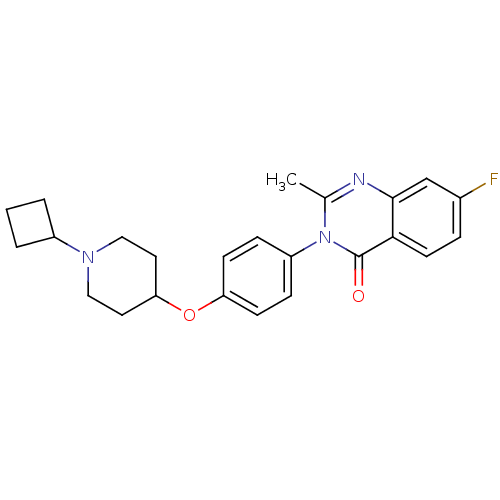
SMILES Cc1nc2cc(F)ccc2c(=O)n1-c1ccc(OC2CCN(CC2)C2CCC2)cc1
InChI Key InChIKey=OTYSUGYIRNYCSI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50274735
Found 2 hits for monomerid = 50274735
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Antagonist activity at human ERG in HEK293 cells assessed as inhibition of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihyd...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT...More data for this Ligand-Target Pair