null
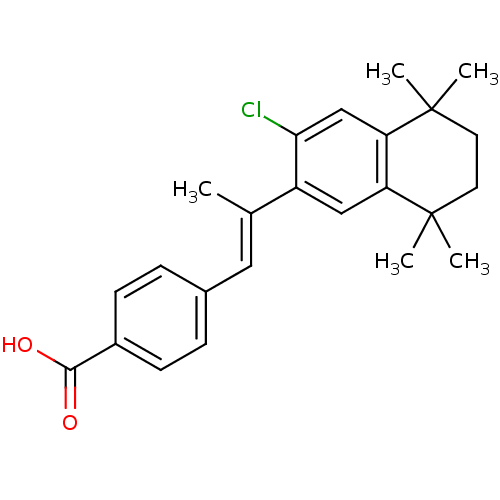
SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1cc2c(cc1Cl)C(C)(C)CCC2(C)C
InChI Key InChIKey=ZMSVHSPBTBNKJJ-NTCAYCPXSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50282693
Found 4 hits for monomerid = 50282693
Affinity DataEC50: 275nMAssay Description:Transcriptional activation for RXR alpha receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Transcriptional activation for RAR beta receptorMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+3nMAssay Description:Transcriptional activation for RAR alpha receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 77nMAssay Description:Transcriptional activation for RAR gamma receptorMore data for this Ligand-Target Pair