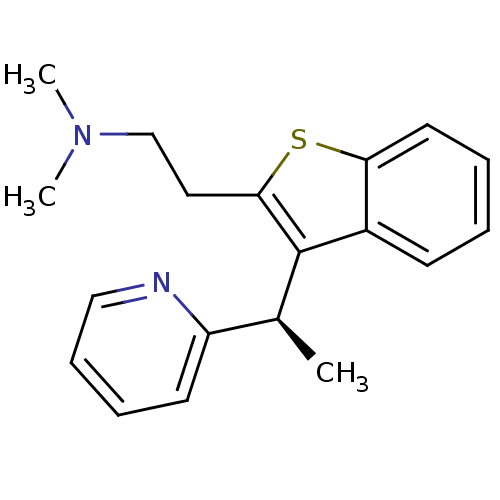
null
SMILES C[C@H](c1c(CCN(C)C)sc2ccccc12)c1ccccn1
InChI Key InChIKey=NANQRVOKMXDMNL-AWEZNQCLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 50297309
Found 18 hits for monomerid = 50297309
Affinity DataKi: 4nMAssay Description:Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of histamine H1 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: >5nMAssay Description:Displacement of [3H]Dofetilide from human ERGMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 5.30nMAssay Description:Displacement of [3H]N-methylscopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of human ERG expressed in HEK cells assessed as blockade of potassium tail current by standard patch clamp analysisMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Displacement of [3H]N-methylscopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: >5.00E+3nMAssay Description:Displacement of [3H]Dofetilide from human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant CYP3A4 after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of recombinant CYP2D6 after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of recombinant CYP2D6 after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataIC50: 5.30E+3nMAssay Description:Displacement of [3H]N-methylscopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant CYP3A4 after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells by patch-clamp techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 2.82E+4nMAssay Description:Inhibition of recombinant CYP2D6 in presence of NADPH generating systemMore data for this Ligand-Target Pair