null
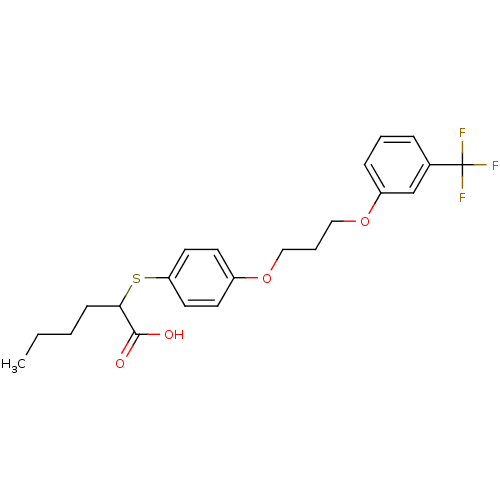
SMILES CCCCC(Sc1ccc(OCCCOc2cccc(c2)C(F)(F)F)cc1)C(O)=O
InChI Key InChIKey=JZYMMZPAPFPFPX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50297886
Found 5 hits for monomerid = 50297886
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 25nMAssay Description:Agonist activity at human PPARalpha expressed in african green monkey COS7 cells by Gal4 transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 4.60E+3nMAssay Description:Agonist activity at human PPARgamma expressed in african green monkey COS7 cells by Gal4 transactivation assayMore data for this Ligand-Target Pair
TargetProstaglandin E synthase(Homo sapiens (Human))
Eberhard Karls University Tuebingen
Curated by ChEMBL
Eberhard Karls University Tuebingen
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 minsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Eberhard Karls University Tuebingen
Curated by ChEMBL
Eberhard Karls University Tuebingen
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Eberhard Karls University Tuebingen
Curated by ChEMBL
Eberhard Karls University Tuebingen
Curated by ChEMBL
Affinity DataIC50: 9.80E+3nMAssay Description:Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a...More data for this Ligand-Target Pair