null
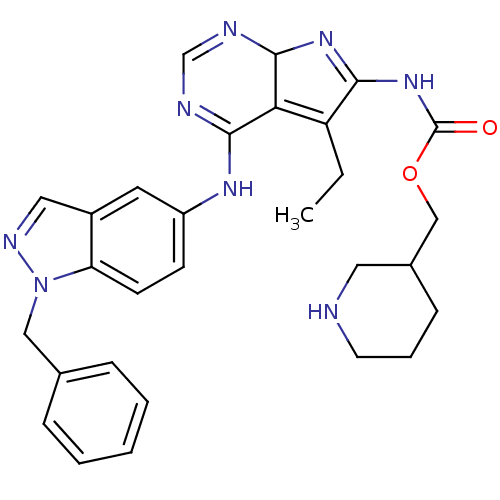
SMILES CCC1=C2C(N=C1NC(=O)OCC1CCCNC1)N=CN=C2Nc1ccc2n(Cc3ccccc3)ncc2c1
InChI Key InChIKey=IZUJRRQQMYQCBU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50299494
Found 2 hits for monomerid = 50299494
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of human recombinant HER1 expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of human recombinant HER2 expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair