null
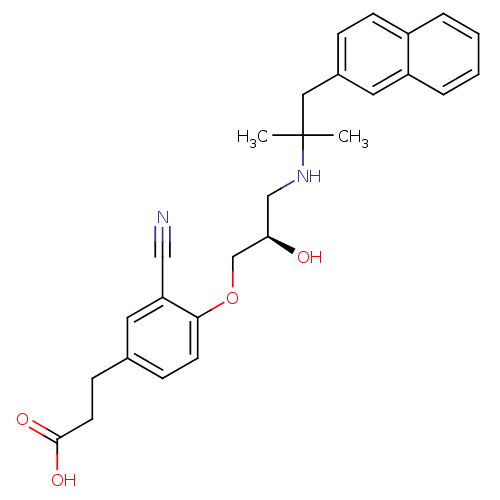
SMILES CC(C)(Cc1ccc2ccccc2c1)NC[C@@H](O)COc1ccc(CCC(O)=O)cc1C#N
InChI Key InChIKey=BKMZXLKUUMOTKA-XMMPIXPASA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50299546
Found 8 hits for monomerid = 50299546
Affinity DataIC50: 30nMAssay Description:Inhibition of beta2 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0220nMAssay Description:Displacement of [3H](2-(2-hydroxyphenyl)-6-methyl-5-(2-methylpropyl)-3-(2-phenylethyl)-4(3H)-pyrimidinone) from human calcium receptor expressed in h...More data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of NETMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of DATMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Antagonist activity at human calcium receptor expressed in HEK293 4.0-7 cells assessed as inhibition of intracellular calcium release by FLIPR assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair