null
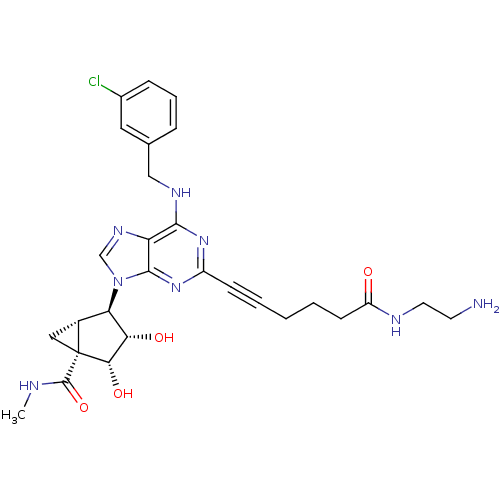
SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCc3cccc(Cl)c3)nc(nc12)C#CCCCC(=O)NCCN
InChI Key InChIKey=YREVRKNBIMSBSA-HRKXUHDCSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50300285
Found 2 hits for monomerid = 50300285
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 2.17nMAssay Description:Displacement of [125I]AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells after 60 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 454nMAssay Description:Displacement of [3H]CCPA from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by liquid scintillation countingMore data for this Ligand-Target Pair