null
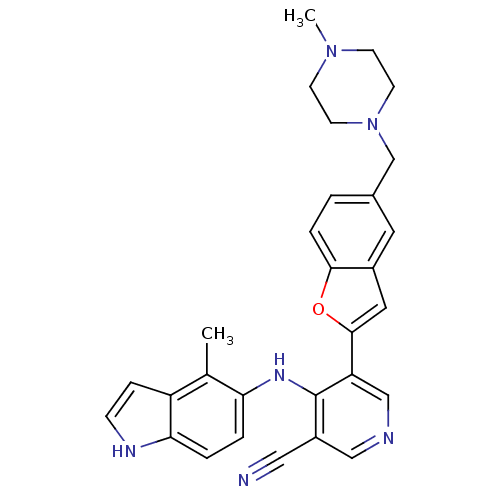
SMILES CN1CCN(Cc2ccc3oc(cc3c2)-c2cncc(C#N)c2Nc2ccc3[nH]ccc3c2C)CC1
InChI Key InChIKey=DZIUPOCVDSYPSY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 50302042
Found 18 hits for monomerid = 50302042
Affinity DataIC50: 33nMAssay Description:Inhibition of PKCeta by IMAP fluorescence polarization technologyMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of PKCtheta in C57BL/6 mouse T cells assessed as inhibition of anti-CD3 and anti-CD28-induced IL2 production after 20 to 24 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibition of human PKCtheta by IMAP fluorescence polarization technologyMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of PKCdelta by IMAP fluorescence polarization technologyMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of PKCbeta by IMAP fluorescence polarization technologyMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of PKCepsilon by IMAP fluorescence polarization technologyMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PKCzeta by IMAP fluorescence polarization technologyMore data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of MK2More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of ROCK1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair