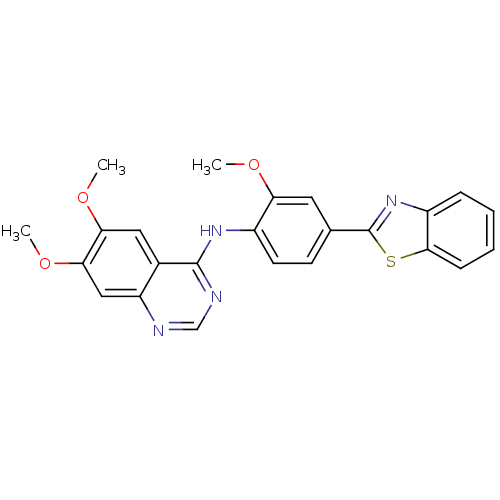
null
SMILES COc1cc(ccc1Nc1ncnc2cc(OC)c(OC)cc12)-c1nc2ccccc2s1
InChI Key InChIKey=HPJSNEQIUZAEBL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50304206
Found 4 hits for monomerid = 50304206
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Aurora-B by microplate scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Inhibition of wild type EGF-R by microplate scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of VEGFR2 by microplate scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Aurora-A by microplate scintillation countingMore data for this Ligand-Target Pair