null
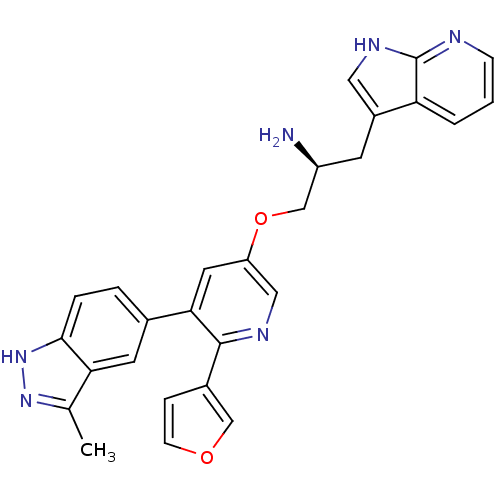
SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ncccc23)cnc1-c1ccoc1
InChI Key InChIKey=FJMIJFHXLDAZDQ-FQEVSTJZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50306093
Found 2 hits for monomerid = 50306093
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of full length AKT1More data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 930nMAssay Description:Inhibition of AKT1 in human BT474 cells assessed as phosphorylation of GSK3More data for this Ligand-Target Pair