null
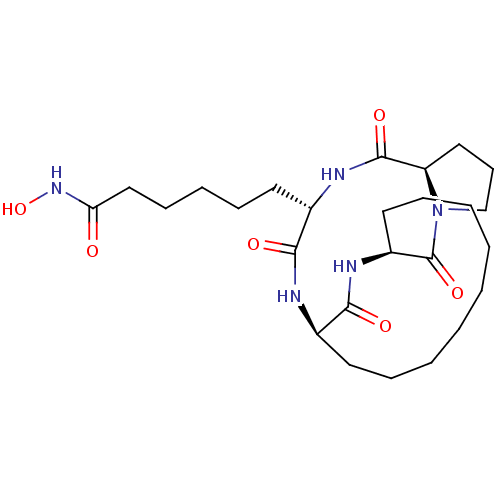
SMILES ONC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@@H]2CCCCCCCCC[C@@H](NC1=O)C(=O)N2
InChI Key InChIKey=QGARLIKKHCLNGY-IVAOSVALSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50309929
Found 3 hits for monomerid = 50309929
TargetHistone deacetylase 1(Homo sapiens (Human))
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
TargetHistone deacetylase 6(Homo sapiens (Human))
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
TargetHistone deacetylase 4(Homo sapiens (Human))
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL
Graduate School of Life Science and Systems Engineering
Curated by ChEMBL