null
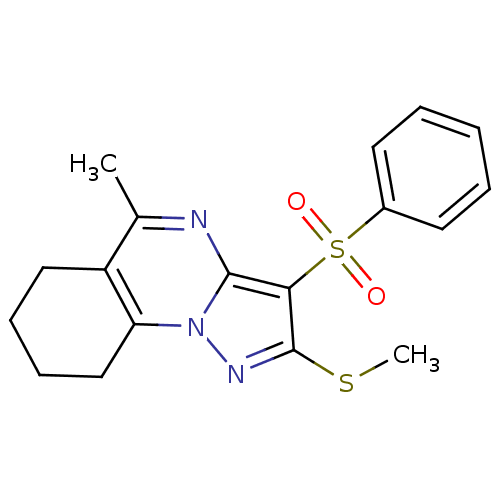
SMILES CSc1nn2c3CCCCc3c(C)nc2c1S(=O)(=O)c1ccccc1
InChI Key InChIKey=NQIHLPFBLRKBRZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50314762
Found 7 hits for monomerid = 50314762
Affinity DataKi: 0.440nM ΔG°: -13.0kcal/mole IC50: 0.948nMpH: 7.4 T: 2°CAssay Description:Determination of tested compounds binding with 5-HT6 receptors was carried out according to the method described in [Monsma F J Jr, Shen Y, Ward R P,...More data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Displacement of [3H]LSD from 5HT6 receptor in humanHeLa cells after 120 minsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2B(Homo sapiens (Human))
Chemical Diversity Research Institute
Curated by ChEMBL
Chemical Diversity Research Institute
Curated by ChEMBL
Affinity DataIC50: 161nMAssay Description:Antagonist activity at human 5HT2B receptor in HEK293 cells assessed as inhibition of alphaME-5-HT-induced cAMP accumulation pretreated for 15 secs b...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Chemical Diversity Research Institute
Curated by ChEMBL
Chemical Diversity Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.40E+3nMAssay Description:Inhibition of human recombinant ERG by patch clamp assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Antagonist activity at 5HT6 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Compounds of general formulas 1 and 2 were tested for their ability to prevent 5-HT6 receptors activation by serotonin. HEK 293 cells (cells of human...More data for this Ligand-Target Pair
Affinity DataIC50: 16.8nMAssay Description:Antagonist activity at human 5HT6 receptor in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation pretreated for 15 mins befor...More data for this Ligand-Target Pair