null
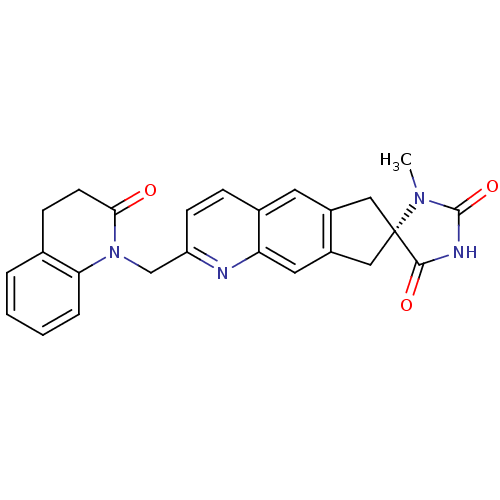
SMILES CN1C(=O)NC(=O)[C@@]11Cc2cc3ccc(CN4C(=O)CCc5ccccc45)nc3cc2C1
InChI Key InChIKey=YXAAGFHRLUCKJU-VWLOTQADSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50315398
Found 3 hits for monomerid = 50315398
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Merck& Co.
Curated by ChEMBL
Merck& Co.
Curated by ChEMBL
Affinity DataKi: 8.90nMAssay Description:Displacement of [125I]human CLR from human CGRP expressed in HEK293 cells coexpressing human RAMP1More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Merck& Co.
Curated by ChEMBL
Merck& Co.
Curated by ChEMBL
Affinity DataIC50: 850nMAssay Description:Antagonist activity at human CLR expressed in human HEK293 cells coexpressing human RAMP1 assessed as Inhibition of CGRP-induced cAMP production in t...More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Merck& Co.
Curated by ChEMBL
Merck& Co.
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Antagonist activity at human CLR expressed in human HEK293 cells coexpressing human RAMP1 assessed as Inhibition of CGRP-induced cAMP productionMore data for this Ligand-Target Pair