null
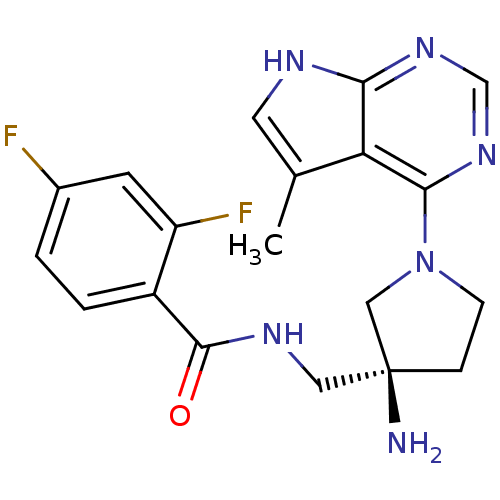
SMILES Cc1c[nH]c2ncnc(N3CC[C@](N)(CNC(=O)c4ccc(F)cc4F)C3)c12
InChI Key InChIKey=BAYGQIFIHYGSEE-IBGZPJMESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50322395
Found 2 hits for monomerid = 50322395
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 310nMAssay Description:Inhibition of AKT1-mediated GSK3alpha phosphorylation in human U87 cells after 1 hr by ELISAMore data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of AKT1 after 90 mins by IMAP assayMore data for this Ligand-Target Pair