null
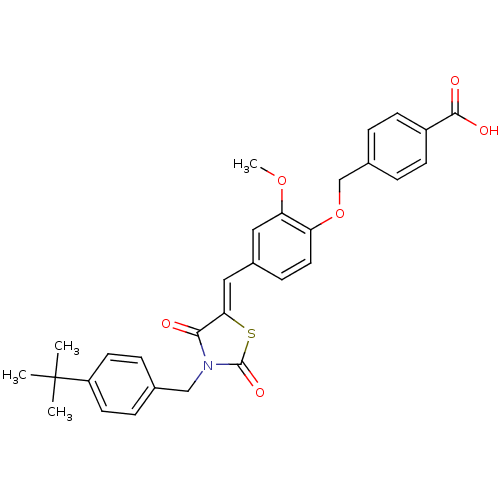
SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(C)(C)C)C2=O)ccc1OCc1ccc(cc1)C(O)=O
InChI Key InChIKey=ABGXPXJMRRMUKP-QQXSKIMKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50322702
Found 2 hits for monomerid = 50322702
TargetEctonucleotide pyrophosphatase/phosphodiesterase family member 2(Homo sapiens (Human))
Institute
Curated by ChEMBL
Institute
Curated by ChEMBL
Affinity DataIC50: 1.36E+4nMAssay Description:Inhibition of 6x-histidine-tagged human recombinant ATX expressed in HEK293 cells assessed as lysophosphatidulcholine releaseMore data for this Ligand-Target Pair
TargetEctonucleotide pyrophosphatase/phosphodiesterase family member 2(Homo sapiens (Human))
Institute
Curated by ChEMBL
Institute
Curated by ChEMBL
Affinity DataIC50: 1.82E+4nMAssay Description:Inhibition of 6x-histidine-tagged human recombinant ATX expressed in HEK293 cells using bisP-nitrophenyl phosphate as a substrateMore data for this Ligand-Target Pair