null
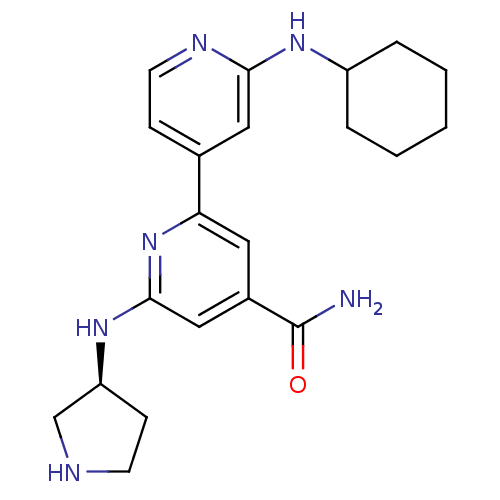
SMILES NC(=O)c1cc(N[C@H]2CCNC2)nc(c1)-c1ccnc(NC2CCCCC2)c1
InChI Key InChIKey=WXGCJNLWHYWDFU-KRWDZBQOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50324336
Found 3 hits for monomerid = 50324336
TargetSerine/threonine-protein kinase D1(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 68nMAssay Description:Inhibition of PKD1 by TR-FRET assayMore data for this Ligand-Target Pair
TargetPolycystin-2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Inhibition of PKD2 by TR-FRET assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase D1(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Inhibition of PKD1 assessed as inhibition of HDAC5 neuclear exportMore data for this Ligand-Target Pair