null
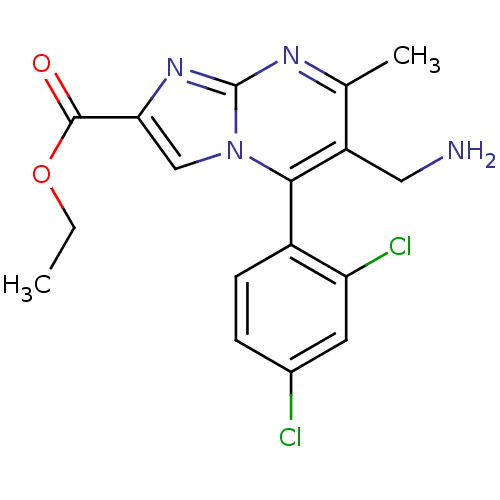
SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl
InChI Key InChIKey=IXOXZCSVVMIYCV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50324514
Found 10 hits for monomerid = 50324514
Affinity DataKi: 0.700nMAssay Description:Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavageMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavageMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Inhibition of human DPP8More data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Inhibition of human DPP8More data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Inhibition of human DPP9More data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Inhibition of human DPP9More data for this Ligand-Target Pair
Affinity DataKi: 11.7nMAssay Description:Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavageMore data for this Ligand-Target Pair
Affinity DataKi: >30nMAssay Description:Inhibition of human DPP8More data for this Ligand-Target Pair
Affinity DataKi: >30nMAssay Description:Inhibition of human DPP9More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The HypoGen module in DS2.5 was employed to produce pharmaphores with the training set compounds.More data for this Ligand-Target Pair