null
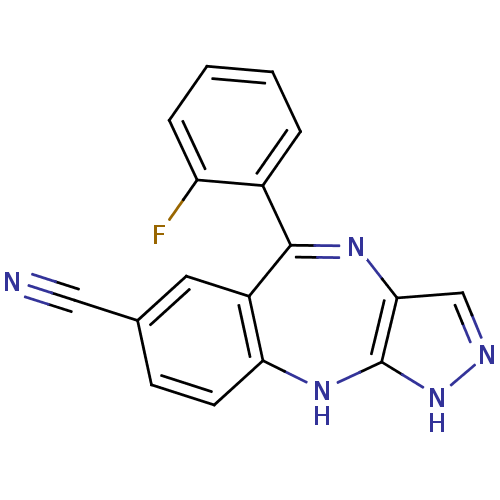
SMILES Fc1ccccc1C1=Nc2cn[nH]c2Nc2ccc(cc12)C#N
InChI Key InChIKey=HVAQMHFWXFSGJF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50328256
Found 5 hits for monomerid = 50328256
Affinity DataIC50: 1.09E+4nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2(Homo sapiens (Human))
Hoffmann-La Roche Inc.
Curated by ChEMBL
Hoffmann-La Roche Inc.
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of CDK2-cyclin EMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Hoffmann-La Roche Inc.
Curated by ChEMBL
Hoffmann-La Roche Inc.
Curated by ChEMBL
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Hoffmann-La Roche Inc.
Curated by ChEMBL
Hoffmann-La Roche Inc.
Curated by ChEMBL
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of SrcMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of FAKMore data for this Ligand-Target Pair