null
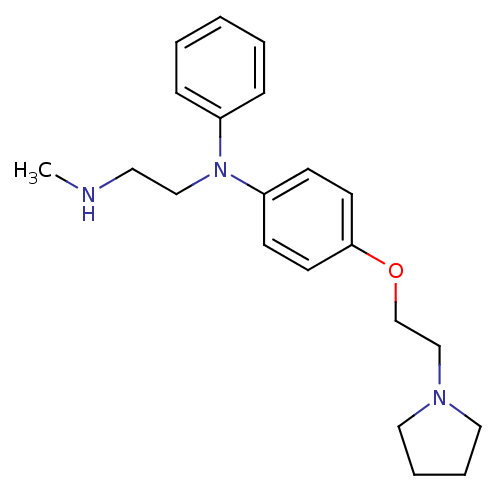
SMILES CNCCN(c1ccccc1)c1ccc(OCCN2CCCC2)cc1
InChI Key InChIKey=LBBBRPSDINTRFT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50330788
Found 4 hits for monomerid = 50330788
Affinity DataKi: 1.60nMAssay Description:Displacement of [3H]-N-R-methylhistamine from human H3 receptor isolated from C6 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.40nMAssay Description:Displacement of [3H]-N-R-methylhistamine from rat H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Displacement of [3H]nisoxetine from rat NET in rat cerebral cortexMore data for this Ligand-Target Pair